![]() The information provided by our expert should not constitute a diagnosis of your condition. Always consult a medical practitioner or healthcare provider for a formal diagnosis. By making use of this content, you agree that ConceiveEasy and the expert assume no liability.
The information provided by our expert should not constitute a diagnosis of your condition. Always consult a medical practitioner or healthcare provider for a formal diagnosis. By making use of this content, you agree that ConceiveEasy and the expert assume no liability.
Could a new test be responsible for revolutionizing the way that the world thinks about IVF? Many scientists and researchers think this could be the case, and it’s all thanks to Eeva, the Early Embryo Viability Test. Eeva is a new investigational test that is currently only available in select European countries. Claim Your 20 Free Pregnancy Tests – Click Here
The test is designed to improve the outcome of IVF treatments and help give couples the best possible chance to become pregnant. Dr. David Walsh of the Sims IVF Clinic in Ireland calls the Eeva test “the most significant breakthrough in IVF in recent decades”, but could it really be possible? Read on for more information about this groundbreaking test.

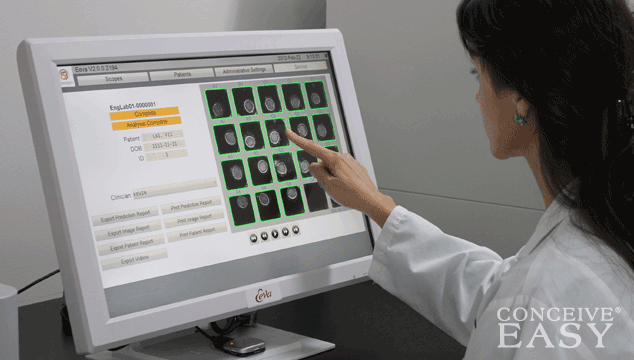
The Eeva test is non invasive, and doesn’t hurt at all. Eeva’s software is able to analyze embryos and classify them as either “high” or “low”, depending on the embryo’s viability. This allows doctors to determine which embryos are most likely to be viable, and which ones are not so great.
The Eeva test is able to use scientific evidence to determine which embryos are most likely to succeed, and it does so with up to 85 percent accuracy. 85% accuracy might not sound like a great percentage, but compared to what doctors and researchers have used before Eeva.
The Eeva test is usually done on Day 3. During the Eeva test, a special microscope will take video of the embryos, and the special Eeva software will analyze the data. Then, Eeva will predict which embryos are most likely to succeed.
Eeva is currently only available in Europe, but there are several investigational clinical trials taking place for Eeva in the United States, and Eeva is pending FDA clearance here in the USA. However, the first Eeva babies are currently being born in Europe, and Eeva is providing a ton of hope for many families out there. “We are proud to be part of a process that can help to give couples a better chance of conceiving children through IVF.” said Lissa Goldenstein, president and CEO of Auxogyn Inc.
The Eeva test has been developed specifically to improve embryo selection before implantation by providing clinicians with information on embryo viability. With more than 500 patients’ embryos tested with Eeva since its approval for use in the EU in July 2012, this successful birth is the first of many more babies to be born throughout Europe.” said Lissa Goldenstein. “We are very excited to rapidly bring this new test to IVF clinics throughout Europe, enabling more families to fulfill their dream in building a family.”








Comments